GANDHINAGAR: A new study led by Rutgers has found that one brain protein, called cypin, plays a key role in learning and memory. This discovery could open new ways to treat serious conditions like Alzheimer’s, Parkinson’s, and brain injuries.
Deciphering Cypin’s Core Functions
For decades, scientists have grappled with the intricate mechanisms that govern the brain’s remarkable ability to learn and remember. At the heart of this complexity lies the synapse, the microscopic gap where brain cells, known as neurons, communicate.
Maintaining robust connections at these synapses is absolutely critical for healthy brain function. Recent research, primarily spearheaded by Distinguished Professor Bonnie Firestein and her team at Rutgers University-New Brunswick, has finally deciphered a pivotal role played by a long-studied brain protein called cypin.
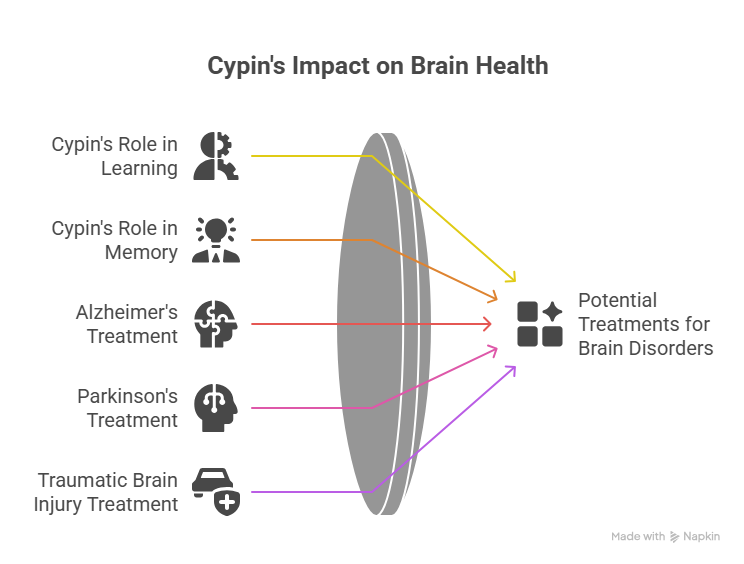
Dr Firestein, who has dedicated over two decades to studying cypin, emphasizes its significance for overall brain health. Her latest findings reveal three key mechanisms through which cypin operates, orchestrating the precise assembly and maintenance of synaptic components vital for neuronal communication.
Precision Tagging: Ensuring Proteins are in the Right Place
One of cypin’s crucial discoveries is its ability to add specialized “localization tags” to proteins within the synapses. This tagging mechanism is akin to attaching exact address labels to packages in a postal system. It ensures that the necessary functional proteins are accurately delivered and positioned at their designated work sites within the synapse, allowing these tiny communication hubs to function properly.
Without this precise marking, synaptic communication would be severely hampered, disrupting the flow of information essential for learning and memory.
The “Intelligent Brake” on Protein Degradation
Another profound discovery illuminates cypin’s interaction with the proteasome, the cellular machinery responsible for breaking down and recycling proteins. When cypin attaches or binds to the proteasome, it acts as a “moderate brake,” slowing down the degradation rate of specific proteins. This sophisticated regulation allows proteins crucial for effective neuronal communication to accumulate within the synapse. This carefully controlled accumulation is vital, as it ensures a sufficient supply of these proteins, bolstering the communication network between neurons.
Dual Upgrade: Boosting Brain Communication
Cypin’s influence extends beyond merely preserving proteins. Firestein’s research shows that an increased presence of cypin directly correlates with higher levels of important proteins in the synapses. These proteins are indispensable for the effective communication between neurons, which, in turn, empowers learning and memory capabilities.
Furthermore, cypin actively increases the activity of another protein called UBE4A, which also plays a role in the critical protein tagging process. Researchers describe this synergistic effect as simultaneously upgrading both the “hardware” (key protein levels) and “software” (UBE4A activation) of the brain’s communication infrastructure. This dual action substantially boosts the efficiency and reliability of information transmission between brain cells, underpinning robust cognitive function.
Beyond these specific mechanisms, cypin (also known as GDA, or guanine deaminase) plays a broader role. It is the primary guanine deaminase in the brain, activating the purine salvage pathway to produce uric acid, a known antioxidant that can be beneficial after brain injury. Cypin also regulates dendrite number and morphology, influencing how neurons integrate information and promoting microtubule assembly. A reduced number of dendrite branches is observed in learning disorders and neurodegenerative conditions like Alzheimer’s.
Bridging Basic Science to Clinical Hope
The implications of these discoveries are profound, offering a revolutionary new approach for treating a range of debilitating neurological conditions. The study, published in the esteemed journal Science Advances, points the way to new treatments for traumatic brain injuries (TBI) and neurodegenerative diseases such as Parkinson’s and Alzheimer’s.
These diseases commonly feature the damage and loss of neuronal connections, leading to declines in memory, cognition, and motor functions.
“Our research indicates that developing treatments or therapies that specifically focus on the protein cypin may help improve the connections between brain cells, enhancing memory and thinking abilities,” states Professor Firestein.
This suggests that cypin could be directly leveraged to develop treatments for neurodegenerative and neurocognitive diseases, as well as brain injuries.
A crucial aspect of cypin’s therapeutic potential lies in its role in synaptic plasticity—the brain’s innate ability for synapses to strengthen or weaken over time. This adaptability is the very basis of learning and memory.
By promoting synaptic plasticity, cypin may be used to counteract the synaptic dysfunction frequently observed in neurodegenerative diseases and brain injuries. When these diseases cause “signal disruption” in the brain, therapies based on modulating cypin protein function hold promise as powerful potential means to rebuild the brain cell “communication network”.
Professor Firestein emphasizes that while this is “basic research,” it has clear pathways to be applied in practical, clinical settings. She is already engaged in “translational” work in parallel, which is the process of taking laboratory discoveries and transforming them into practical treatments or solutions to improve human health.
The Experiment: How Scientists Unlocked Cypin’s Secrets
The research leading to these breakthroughs was a collaborative effort led by Rutgers University-New Brunswick. The study was supported in part by the National Institutes of Health (NINDS), the Coalition for Brain Injury Research, a charitable foundation, and private donors.
Key contributors from Rutgers included Kiran Madura, Srinivasa Gandu, Mihir Patel, and Ana Rodriguez, with additional contributions from Jared Lamp and Irving Vega of Michigan State University.
The team employed a multi-faceted approach to uncover cypin’s functions and validate its therapeutic potential.
In Vitro Insights: Protecting Neurons from Damage
Initial investigations focused on understanding cypin’s role in neuroprotection following injury. Using an in vitro model of secondary brain injury, where neurons were exposed to NMDA (N-methyl-D-aspartate) to induce excitotoxicity, the researchers observed significant neuronal loss.
However, pretreatment with newly identified small molecule activators of cypin, specifically H9 and G5, resulted in 100% neuronal survival after NMDA-induced injury, compared to 50% survival in untreated cultures. In contrast, a cypin inhibitor, B9, showed no beneficial effect and even caused neuronal death on its own.
Electrophysiological studies on primary hippocampal neurons further revealed cypin’s pre- and postsynaptic roles. Cypin knockdown increased the amplitude of miniature excitatory postsynaptic currents (mEPSCs), while cypin overexpression increased their frequency.
This suggests cypin influences both neurotransmitter release from the presynaptic side and receptor composition at the postsynaptic side. Importantly, cypin activators (H9 and G5) reversed the electrophysiological changes normally seen after NMDA-induced injury, maintaining mEPSC frequency and amplitude. These protective effects were dependent on cypin expression, as the activators could not confer protection when cypin was knocked down.
In Vivo Validation: Restoring Memory After Brain Trauma
The most compelling findings came from in vivo studies using a controlled cortical impact (CCI) model of traumatic brain injury (TBI) in mice. Mice subjected to moderate CCI exhibited significant deficits in fear conditioning, a behavioral test used to evaluate contextual memory.
One hour after injury, the scientists directly injected the small molecule compounds into the ipsilateral hippocampus of the mice. Treatment with cypin activators H9 or G5 significantly improved the freeze percentage in the fear conditioning test, compared to untreated or vehicle-treated injured animals. In fact, for G5, the treated injured animals showed no significant difference in fear conditioning from sham (uninjured) animals.
Conversely, treatment with the cypin inhibitor B9 offered no improvement and even impaired sham animals. This provided the first direct evidence that cypin activation can promote cognitive recovery after in vivo traumatic brain injury.
The researchers also investigated whether the compounds affected cypin or PSD-95 protein levels in vivo. While H9 led to a transient elevation in PSD-95 in the cortex, none of the compounds significantly changed cypin levels, suggesting their neuroprotective effects are primarily through modulating cypin’s enzymatic activity rather than its overall abundance.
A New Frontier in Brain Treatment
The discovery that cypin activation offers neuroprotection and promotes cognitive recovery marks a significant advance in the search for TBI and neurodegenerative disease treatments. While synaptic plasticity is known to aid recovery after injury, there have been few direct therapeutic approaches to manipulate the proteins that actively rebuild damaged neural circuits.
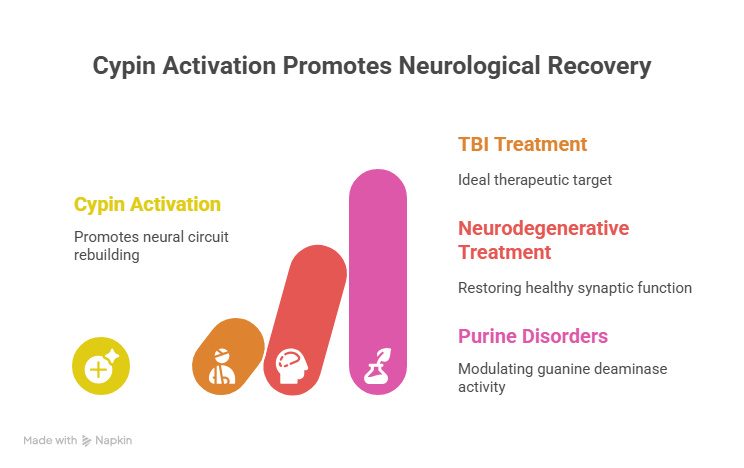
Cypin stands out as an ideal therapeutic target for TBI. Unlike other proteins that have been explored, such as PSD-95 or calcineurin, cypin is the primary guanine deaminase in the brain and can influence both dendrites and dendritic spines, as well as pre- and postsynaptic mechanisms.
This multi-faceted role suggests that targeting cypin could offer a more comprehensive approach to restoring brain function. For example, healthy synaptic function is often disrupted in diseases like Alzheimer’s and Parkinson’s, making cypin’s role in maintaining these connections particularly relevant.
The identified activators, which are N-benzoyl piperazines, open up new possibilities for drug development. The fact that cypin levels don’t significantly decrease after trauma indicates a potentially wider therapeutic window for these small molecules.
Beyond TBI and neurodegenerative diseases, the ability to modulate cypin’s guanine deaminase activity could have broader utility for other purine metabolic disorders with neurological consequences, such as Lesch-Nyhan Syndrome. These disorders often involve abnormal purine metabolism that affects neuronal development, including dendrite outgrowth and branching.
Future studies will focus on defining the optimal therapeutic window for cypin modulation after injury, assessing the efficacy of these activators across different injury severities, and continually refining the compounds to enhance their mechanism of action and improve their ability to cross the blood-brain barrier.
This research not only deepens our fundamental understanding of how the brain works but also ignites new hope for developing novel strategies to combat devastating neurological diseases, paving the way for a future where brain trauma and neurodegeneration might be treated more effectively than ever before.


